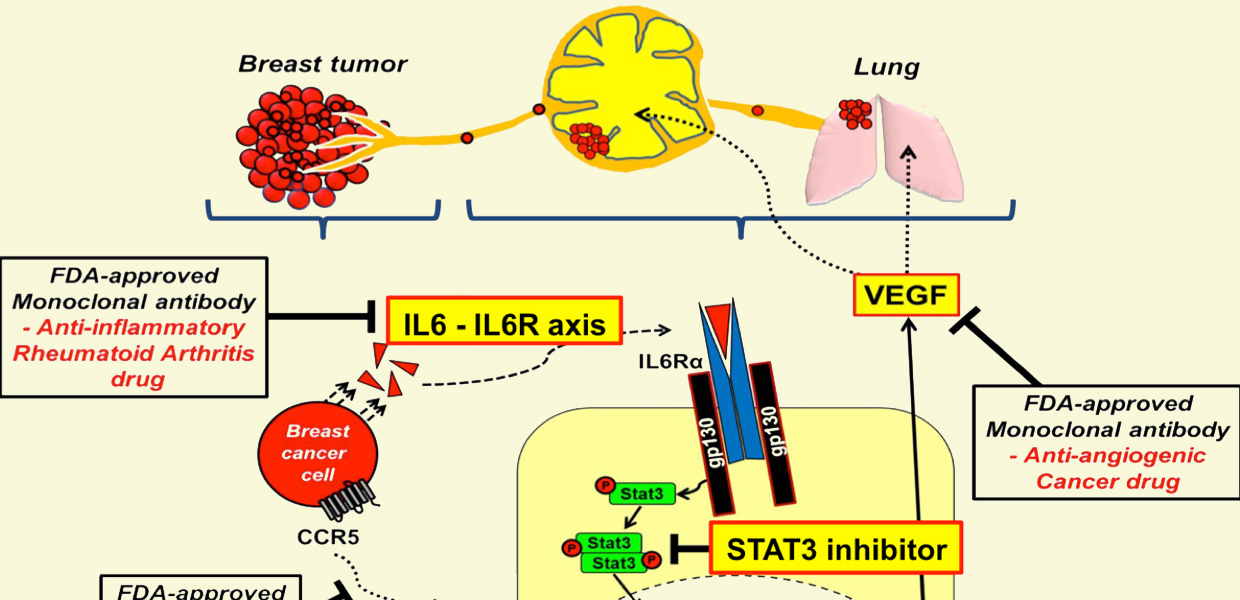
Schematic of FDA approved drug blocking breast cancer metastasis. Popel Lab / JHU
Lymphatic endothelial cells play a key role in orchestrating metastatic colonization of such organs as lymph nodes and lungs in breast cancer, Johns Hopkins researchers have found in their preclinical mouse research; they also identified FDA-approved drugs used for other indications (repurposed drugs) that could be used to inhibit or prevent metastasis. This is very important because metastasis is the major cause of death in cancer patients.
These findings published in the September 2, 2014 online edition of Nature Communications in a paper entitled “Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis” contribute significantly to our understanding of the role of stromal, non-cancer cells in the development of metastasis and shows possible routes to treatment. The identification of drugs previously approved for other indications, such as HIV and rheumatoid arthritis, opens attractive possibilities for treating cancer patients. Currently, it takes 10-12 years and billions of dollars to develop a new drug from discovery to market; however, using repurposed or repositioned drugs promises significant savings in time and resources.
The team of investigators led by Aleksander S. Popel, PhD, professor in the Department of Biomedical Engineering in the School of Medicine, with joint appointments in the Department of Oncology and the Sidney Kimmel Comprehensive Cancer Center, found that normal lymphatic endothelial cells when subjected to molecular factors secreted by cancer cells are conditioned or reprogrammed, in such a way that they in turn start secreting molecules that facilitate metastasis. The team used a new metastatic mouse model where the metastatic colonization was induced by injections of liquid containing factors secreted by cancer cells (tumor conditioned media, TCM), mimicking the secretion from primary breast tumor. They chose to deal with triple-negative breast cancer which is especially aggressive, metastatic, and difficult to treat. Specifically, they have shown that human triple-negative breast cancer cells secrete a cytokine protein interleukin-6 (IL6) that triggers lymphatic endothelial cells located within lymph nodes and the lungs to secrete a chemokine protein CCL5 and vascular endothelial growth factor VEGF. The CCL5 chemokine then helps recruit cancer cells that express CCL5 receptor CCR5, and VEGF promotes vascular growth (angiogenesis) in lymph nodes and increases vessel permeability in the lungs. Both processes facilitate colonization of these organs with cancer cells leading to metastatic growth.
Having discovered these mechanisms, the researchers then designed experiments in mice that would disrupt the communications between CCL5 and CCR5 using a CCR5 inhibitor maraviroc, an FDA-approved HIV drug, and found a significant inhibition of metastasis. A combination of maraviroc and an anti-VEGF agent completely eliminated metastasis. In addition, depletion of IL6 in the TCM using an IL6 antibody also resulted in complete inhibition of metastasis.
These preclinical studies suggest that similar treatment might lead to new ways of preventing metastasis and treating metastatic patients. For example, IL6 receptor inhibitor tocilizumab is an FDA-approved anti-inflammatory drug and maraviroc or other CCR5 inhibitors could be administered prior or after surgery, possibly in combination with standard of care therapeutics.
These preclinical results were reinforced by bioinformatic analysis of available samples from breast cancer patients that show a significant expression of CCL5 and IL6 in patients with triple-negative breast cancer compared with other types. “We are planning to collaborate with our clinical colleagues to eventually translate our findings to the clinic,” Popel says. “A lot of work lies ahead and a truly multidisciplinary team is needed; the Sidney Kimmel Comprehensive Cancer Center is ideally positioned for such translational work.”
Other Johns Hopkins researchers who participated in this study were Esak Lee, the first author of the paper, Elana J. Fertig, Kideok Jin, Saraswati Sukumar, and Niranjan B. Pandey.
The work was supported by the National Institutes of Health grant R01 CA138264 and the Safeway Foundation for Breast Cancer.
